
| 
|
|
|
|
|
| |
|
|
 08/2024/ A New Photocaging Method for Imidazole Derivatives with Biological Activity. |
|
Photoswitches that play an important role in photopharmacology can be classified into those that show reversible structural changes like cis-trans isomerization of azobenzene derivatives, and those that show one-time, unidirectional structural changes like the photolysis of o-nitrobenzyl ester groups. Most of the latter require ultraviolet light for their photoreaction, and the byproducts generated by the photoreaction were relatively large compounds with aromatic rings, such as benzaldehyde derivatives.
This time, we have discovered a new photocaging method for imidazole derivatives, many of which exhibit physiological activity such as anticancer effects. Caged compounds can be synthesized in a one-step reaction from compounds known to exhibit biological activity, and the photolytic deprotection reaction of the caged compounds can be achieved with blue light irradiation at 405-430 nm. Additionally, the byproducts are low-molecular-weight compounds without aromatic rings, derived from dimethylaminyl radicals.
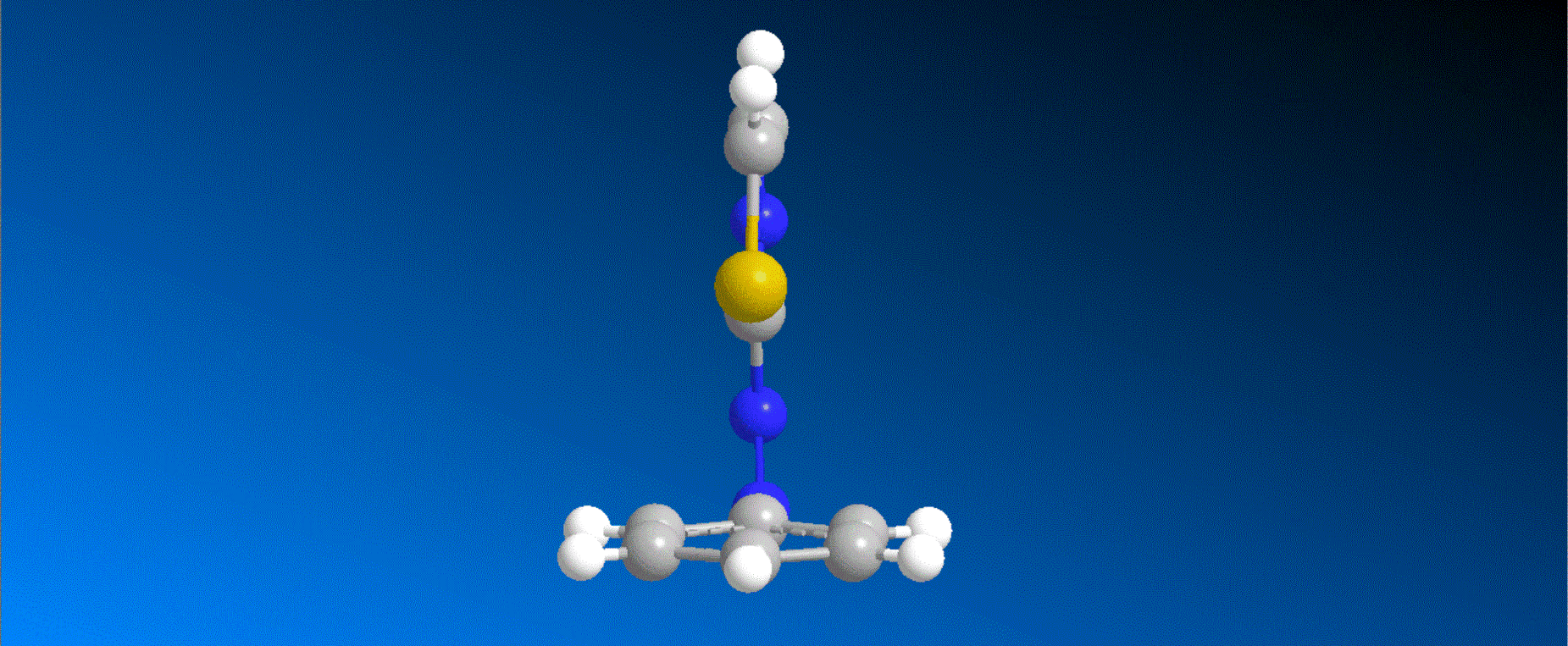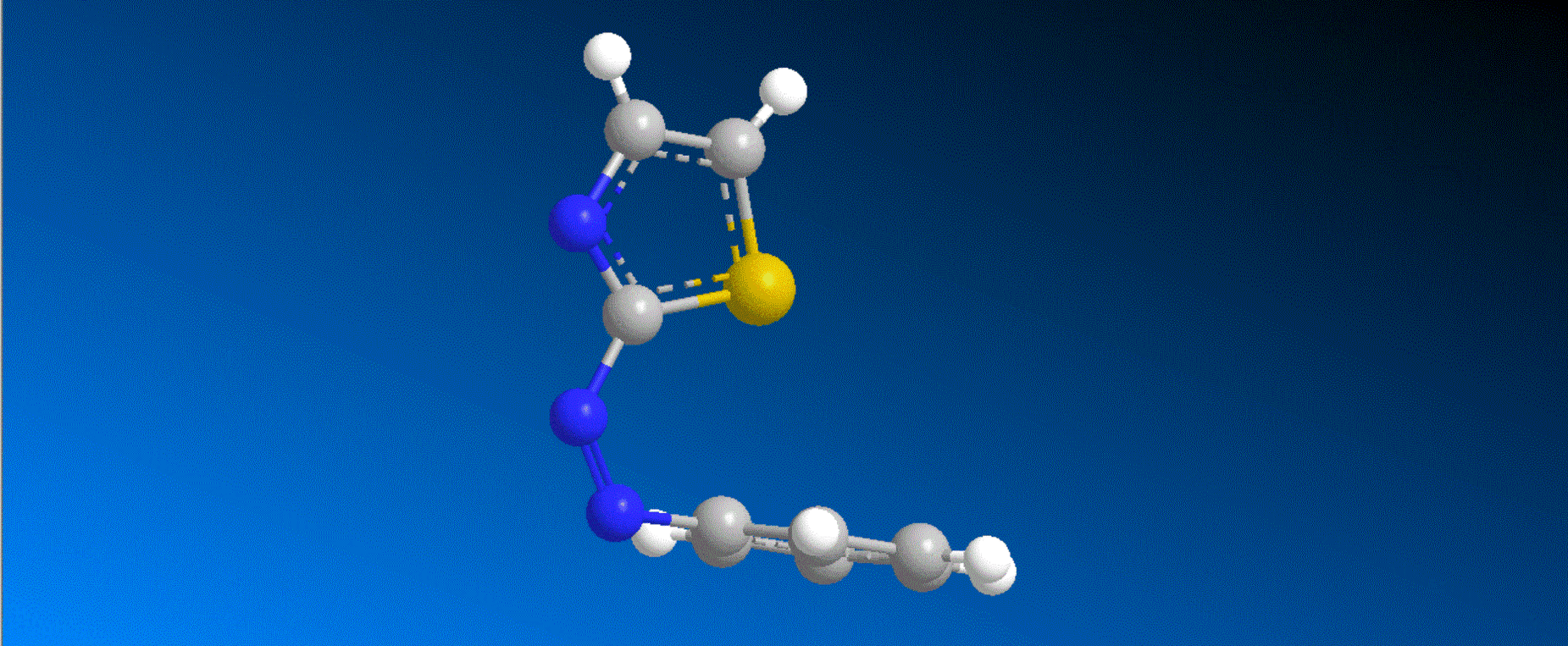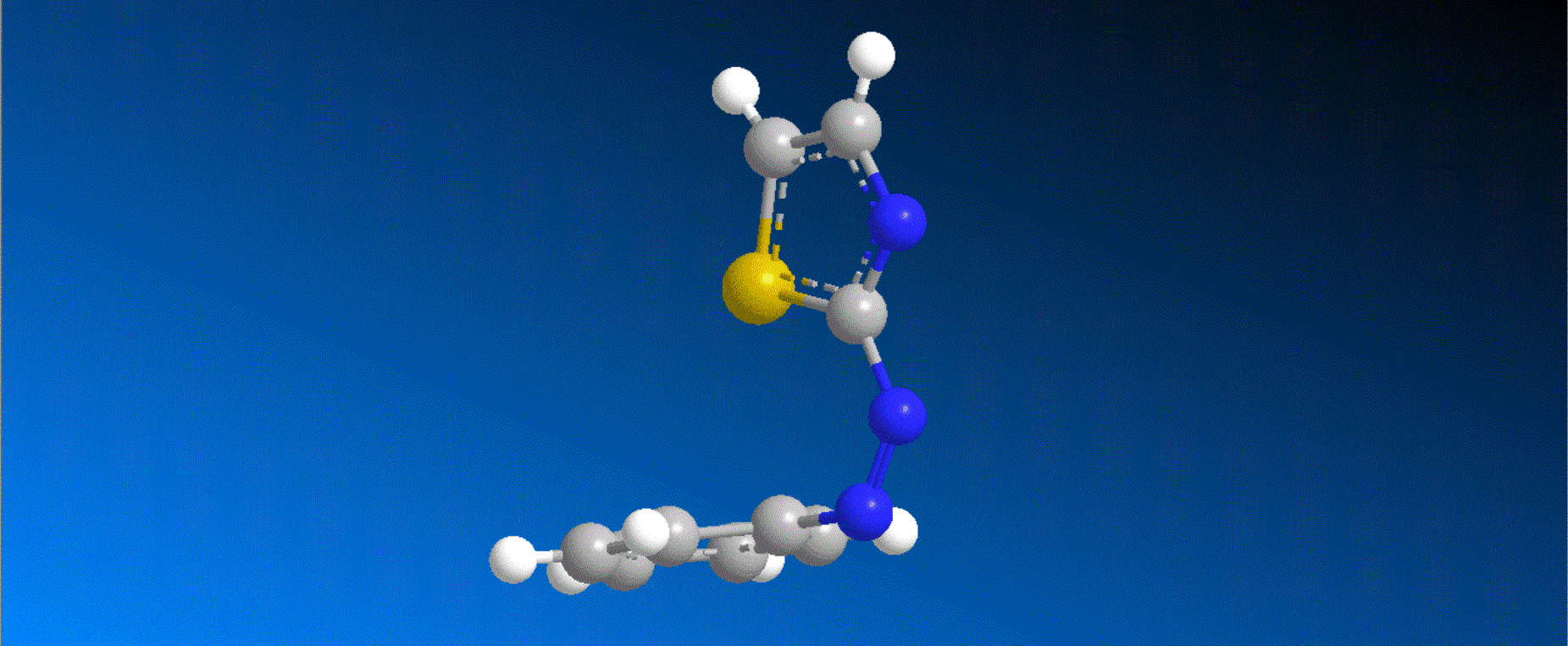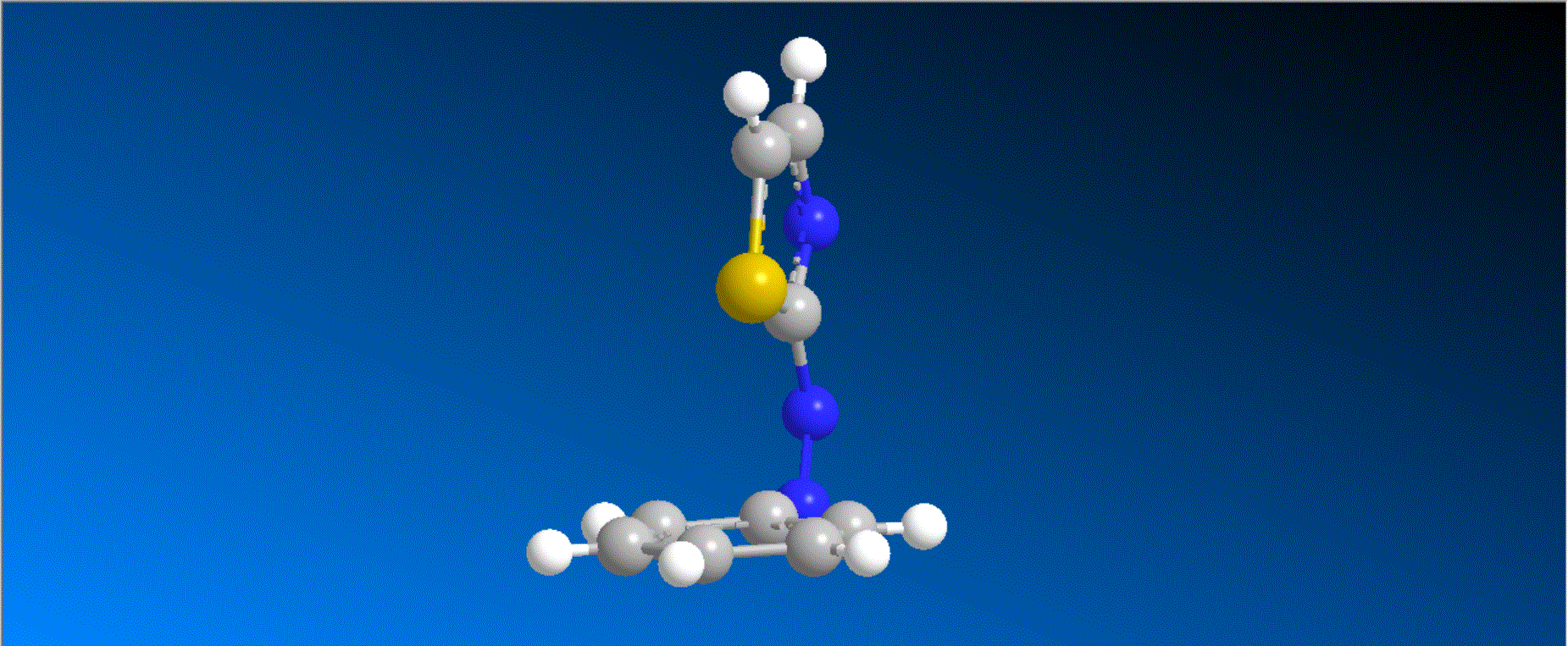
The structure of the caged compounds, which can be obtained in a one-step reaction from imidazole derivatives with kinase inhibitory activity, and the photoreaction scheme in which imidazole with the original biological activity is produced by light irradiation, are shown in the figure above. In this study, we considered caging SB431542, a compound that inhibits kinases related to the cytokine TGF-β signaling pathway, and synthesized the caged compounds 1 and 2 (shown in the figure below).
Both compounds 1 and 2 efficiently produced SB431542 upon irradiation with light at 405-430 nm. In a wound healing assay using human breast cancer cells MDA-MB-231, it was found that the motility of cells to which compound 1 was added was smaller compared to cells to which compound 1 was added and further irradiated with 405 nm light. This result indicates that compound 1, unlike the original kinase inhibitor SB431542, shows almost no kinase inhibition, and that compound 1 quickly changes to SB431542 with kinase inhibitory effects when irradiated with visible light, thereby exhibiting kinase inhibitory effects that slow cell movement.
This study was featured as news on the website "ChemistryViews" and the synthesis and effects of the compound were explained in "Synfacts"Synfacts.
|
| |
|
|
Jiajun Qi, Ammathnadu S. Amrutha, Sumire Ishida-Ishihara, Hisham M. Dokainish, P. K. Hashim, Ryu Miyazaki, Masumi Tsuda, Shinya Tanaka, and Nobuyuki Tamaoki, "Caging Bioactive Triarylimidazoles: An Approach to Create Visible Light-Activatable Drugs""
J. Am. Chem. Soc., 2024, 146, 18002-18010
|
|
|
 11/2020/ We developed a photoresponsive CENP-E inhibitor that enables ON-OFF optical switching in the cell cycle and marketed it as a reagent. |
|
Recently, we have succeeded in completely turning on and off the motor function of motor proteins. As the next step, it is important to control more macroscopic phenomena with molecular switches. In this study, we aimed to synthesize a compound that has a photoresponsive inhibitory effect on motor proteins that control chromosome migration during cell division, and to photoswitch the macroscopic phenomenon of cell division.
This time we aimed at photoresponsive inhibition of the motor protein CENP-E, which controls the transport of chromosomes to the equatorial plane in the so-called M phase. CENP-E inhibitors are currently expected as candidates for anti-cancer drugs that kill cancer cells that undergo frequent cell division. If the inhibitor exhibits photoresponsiveness, cancer cells can be selectively killed by stopping the function of CENP-E and leading to apoptosis by irradiating the cancer patient.
The structure of the synthesized photoresponsive CENP-E inhibitor is shown in the figure (top). This inhibitor inhibited the ATP hydrolysis activity of CENP-E after irradiation with visible light, and the inhibitory activity disappeared after irradiation with ultraviolet light. We also confirmed that chromosome migration can be switched photoreversibly in cancer cells (Fig. (Bottom)). Furthermore, it was confirmed that after irradiation with visible light, it led to cell death due to intracellular CENP-E inhibition, while after irradiation with ultraviolet light, there was no effect on cells. The above results show for the first time the dynamic control of the cell cycle by photochemical reactions. We expect that it not only provides useful tools in cell cycle research, but also triggers the development of anticancer drugs working selectively by the action of lights.
The synthesis of this compound was introduced in the magazine "Synfacts", which introduces the latest synthesis technology.
Funakoshi Co., Ltd. has started selling this compound as a reagent under the trade name of "PCEI-HU".
This research is a joint research with Associate Professor Ryota Uehara, Faculty of Life Sciences, Hokkaido University. This research is supported by the Tokyo Foundation for the Promotion of Science and Technology.
. |
| |
|
|
Noushaba Nusrat Mafy, Kazuya Matsuo, Shota Hiruma, Ryota Uehara, Nobuyuki Tamaoki, "Photoswitchable CENP-E inhibitor enabling the dynamic control of chromosome movement and mitotic progression", J. Am. Chem. Soc., 2020, 142, 1763-1767
|
|
|
 11/2017 Our recent article on photo-regulation of myosin-actin motors with photoresponsive high energy molecules was featured in the inside cover page of Organic and Biomolecular Chemistry. |
|
It is a hot field to make natural molecular motors, namely motor proteins, applicable to nanotechnology especially nano-transportation. In order to accomplish such an application it is important to precisely regulate the motile function of natural motor proteins at molecular and macroscopic scales. In the present work we first confirmed that photo-responsive non-nucleoside triphosphates (AzoTP and its analogues) instead of the original energy molecule adenosine triphosphate (ATP) could drive myosin, one of motor proteins in our body. Furthermore we could control the driving velocity of the molecular motor reversibly by the photoisomerization of an azobenzene group in the non-nucleoside molecule. We also demonstrated the photo-regulation of muscle fibers containing myosin-action motor system using the fore mentioned non-nucleoside molecule. These demonstrations became possible by our original design of the energy molecule where the base aromatic and the ribose units in ATP were exchanged with azobenzene and some linkers, respectively. We believe the present work not only accelerate the progress of the study of soft molecular machines, but also provide a new tool for the study of the mechanism of motor functions of motor proteins.
An illustration summarizing our present accomplishment was used as an inside cover of the issue where our article is included of Organic and Biomolecular Chemistry. |
| |
|
|
Halley M. Menezes, Md. Jahirul Islam, Masayuki Takahashi and Nobuyuki Tamaoki "Driving and photo-regulation of myosin-actin motors at molecular and macroscopic levels by photo-responsive high energy molecules"
Org. Biomol. Chem., 2017, 15, 8894-8903
|
|
|
 11/2016 Single microtubule manipulation by light became possible . |
|
Constructing molecular machines with controllable movements has been an important goal in chemistry and nanotechnology and the pioneers in this field have been recognized with the Nobel Prize in Chemistry this year. Molecular machines will most likely be used in nanomedicine and the development of things such as new materials, sensors and energy storage systems. One way to construct useful molecular machines is to combine natural molecules ? such as proteins or DNAs in our body ? with synthetic molecules in order to control the functions of the natural molecules. Building on previous work that allowed to achieve complete control over ON/OFF switching of the movement of a nanomachine, we developed a molecular system which allows free control of the motion of single microtubules. This new work was featured as Nanotechnology Spotlight in a nanotechnology portal site, Nanowerk.
The figure on the left is a schematic illustration of the transportation of single microtubule on kinesin coated surface in the presence of photoresponsive inhibitor. Under 365 nm local light irradiation selected microtubule was translocated, while almost arresting ambient microtubules under 510 nm. The movie of this event was recorded during observation on a fluorescent optical microscope. |
| |
|
|
K. R. Sunil Kumar, Ammathnadu S. Amrutha and Nobuyuki Tamaoki "Spatiotemporal control of kinesin motor protein by photoswitches enabling selective single microtubule regulations"
Lab Chip, 2016, 4702-4709
|
|
|
 7/2016 Our recent article on the structure-property relationships of photoresponsive inhibitors of the kinesin-1 was featured in the cover page of Organic and Biomolecular Chemistry journal. |
|
Recently we demonstrated the reversible photoregulation of the kinesin motor activity using azobenzene tethered peptide (azo-peptide) composed of eleven amino acids derived from the tail domain of the kinesin ( K. R. Sunil Kumar et al. ACS Nano, 2014, 8(5), 4157-4165). To know the mechanism of the photoswitchable inhibition, structure-property relationship study of the array of azo-peptides was carried out by incorporating the structural modifications at the peptide unit and at the photoresponsive unit. Our study gave the important information about the vital amino acids responsible for the inhibition, types of interactions involved in inducing the inhibition and the substituent group presenting on azobenzene exhibiting higher photoswitchability. As a result, a new, more efficient photoresponsive inhibitor of kinesin featuring relatively short peptide unit (-Arg-Ile-Pro-Lys-Ala-Ile-Arg-OH) coupled to an azobenzene unit substituted with an OMe group at the para position was obtained. This piece of work will be helpful in the molecular designing of the photoresponsive inhibitors for the applications in artificial nanotransportation systems, in the study and treatment of diseases etc. Interaction of the photoresponsive inhibitor with the kinesin motor domain and the reversible isomerization of the azo unit triggered by light are presented in this picture which was featured in the cover page of the journal ' Organic and Biomolecular Chemistry' published by Royal Society of Chemistry. |
| |
|
|
|
|
|
 3/2014 Complete photo-switching of a biomolecular machine was attained |
|
In our body, molecular machines with a nano meter size are working. If we took them out from our body and artificially regulated the motile function, it would lead to the actualization of “flexible and compact machine” which is totally different from the present hard and bulky machines. We developed a new photoresponsive inhibitor for kinesin, one of natural molecular machines, and succeeded in complete ON-OFF photo-switching of the motile property of kinesin by adding the inhibitor.
This work was featured as Nanotechnology Spotlights in a nanotechnology portal site, Nanowerk.
The figure on the left is a schematic drawing of photo-stimulated switching of the movement of microtubules driven by kinesin in the presence of our photoresponsive inhibitor. The movie is showing the movement of microtubules observed on a fluorescent optical microscope. During the observation, 1 sec irradiation each of UV or blue light was applied to switch the state of motility between ON and OFF. The total length of the whole observation was 5 min. |
|
|
| |
|
|
K. R. Sunil Kumar , Takashi Kamei , Tuyoshi Fukaminato , and Nobuyuki Tamaoki "Complete ON/OFF Photoswitching of the Motility of a Nanobiomolecular Machine"
ACS Nano, 2014, 8(5), 4157-4165 |
|
|
We developed a methane derivative having two identical azobenzene substituents and demonstrated that detectable point chirality was generated and ceased upon photo-induced E/Z isomerization of one of the azobenzene substituents. The study may contribute to the study of the origin of enantio-pure molecules in nature and to the development of optical materials regulating polarized lights which is used for 3D displays. This work was featured in ChemViews Magazene as News entitled “Central Chirality by E/Z Photoisomerization” |
|
|
| |
|
|
|
|
|
Our review article on the photochromic regulation of liquid crystals was published in Journal of Photochemistry and Photobiology C as an “Invited Review Paper”. The left is an included schematic drawing by Nishad, a PhD student in our lab showing the entire field of the review. |
|
|
| |
|
|
Schematic representation showing that the combination of photochromic compounds, liquid crystals and the action of light produces various dynamic functions for molecular machines, optical memories, lasers, and light modulators.
|
|
|
|
|
|
|
 03/2010 Our Paper on molecular machines was selected as one of the most accessed
articles |
 11/2008 First planar chiral azobenzene made liquid crystals photo-tunable in full-color range |
|
We synthesized a planar chiral azobenzene, which induces chiral nematic phase in commercially available nematic liquid crystals and the change in the reflection color of the thin film of the chiral nematics from blue to red via green by ultra violet light and vice versa by visible light. Our achivement is featured as a highlight in Nature's web site.
|
|
|
M. Mathews and N. Tamaoki, "Planar Chiral Azobenzenophanes as Chiroptic Switches for Photon Mode Reversible Reflection Color Control in Induced Chiral Nematic Liquid Crystals. J. Am. Chem. Soc., 2008, 130, 11409-11416 |
 01/2008 Our new photochromic compounds now on sale! |
|
A Japanese chemicals company, Tokyo Chemical Industry (TCI), started selling our newly developed photochromic compounds as reagents for laboratory experiments.
(Catalogue number: D3618, D3619) |
|
| |
|
|
|
|
|
|
The compounds having a unique spiro perimidine structure reversibly change their color between pale yellow in the closed form and dark brown (or dark blue) in the open form by the action of lights. Since the photochemically generated open form shows the wide absorption covering whole visible region, they have a potential for the application to the automatic light intensity regulator. The free amino group in the compound makes it possible to be modified in the chemical structure or to be introduced in polymer chains.
1. R. Davis, N. Tamaoki, "Novel Photochromic Spiroheterocyclic Molecules via Oxidation of 1, 8- Diaminonaphthalene", Org. Lett., 2005, 7, 1461-1464.
2. R. Davis, N. Tamaoki, "Modulation of Unconventional Fluorescence of Novel Photochromic Perimidine Spirodimers", Chem. Eur. J., 2007, 13, 626-631.
|
|
|
|
|
|
|
|
|
|
|
|
|
 06/2007 Our study made the cover of Advanced Functional Materials. |
|
Our article on phtorespnonseive azobenzane peptides was published in Adv. Funct. Mater. that featured our work in the cover picture of the issue.
(Vol. 17, 2007) |
|
|
| |
|
|
Y. Matsuzawa, K. Ueki, M. Yoshida, N. Tamaoki, T. Nakamura, H. Sakai, M. Abe
"Assembly and Photoinduced Organization of Mono- and Oligopeptide Molecules Containing an Azobenzene Moiety"
Adv. Func. Mater., 2007, 17, 1507-1514
|
|
|
|
|
Top of Page .jpg) |
|